Abstract
INTRODUCTION: Hematopoietic stem and progenitor cells (HSPCs) support life-long hematopoiesis. A single HSPC can also be at the origin of hematological malignancies, such as Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS). Allogeneic HSCT with the intent to eliminate recipient AML or MDS and at the same time replace recipient HSPC with donor-HSPC and immune cells is a life-saving therapeutic option for many patients. However, chemotherapy (and sometimes in addition gamma-irradiation based conditioning regiments) prior to HSCT are associated with substantial toxicity. Thus, due to benefit-outweighing treatment-related toxicity and mortality, frail, multi-morbid and elderly patients are usually excluded from potentially curative allo-HSCT approaches. For these reasons, more selective preconditioning strategies, leading to residual AML/MDS elimination and creating "space" for incoming HSPCs, are required. Selective targeting of CD117 with monoclonal antibodies has been proposed as a strategy to remove endogenous HSPCs, enabling an effective but mild preconditioning. However, specific conditioning of AML and MDS patients, prior to HSCT, might require a more potent effector cell type. We hypothesized that a CD117 and CD3 binding, T cell engaging and activating antibody construct (CD117xCD3 TEA) with a short half-life might be an ideal means to selectively eliminate CD117-expressing healthy HSPCs and residual CD117-expressing AML or MDS cells prior to allo-HSCT.
METHODS: We cloned and expressed CD117xCD3 TEA in tandem scFv format and produced it by transient gene expression in Chinese hamster ovary cells (CHO-S). The fusion proteins were purified to homogeneity by protein A affinity chromatography. We derived target cell lines with varying surface levels of CD117 (high, medium and low) from CD117 negative parental cell lines HL-60 and MOLM-14 (Myburgh et al., Leukemia, 2020). To assess T cell mediated killing of target cells, we mixed them with human T cells (purified and enriched after negative selection) at varying Effector-to-Target (E:T) cell ratios and added CD117xCD3 TEA at different concentrations. The mixture was incubated and specific killing was quantified via flow cytometry at different time-points.
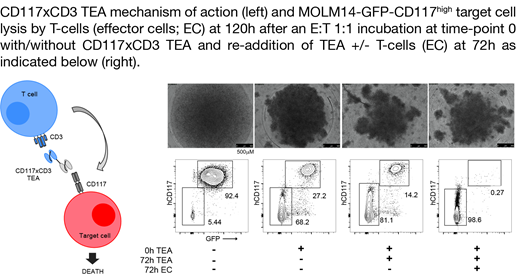
RESULTS: In order to characterize the biocidal properties of CD117xCD3 TEA, we performed in vitro killing experiments against cell lines, HSCPs from healthy donors and blast cells from AML patients. A dose-dependent in vitro killing of the cell lines was observed in the presence of various concentrations of CD117xCD3 TEA and of human T cells at an E:T cell ratio of 10:1 after 24h. The HL60 CD117 high cell line was efficiently lysed (~90%) at 100 ng/ml of CD117xCD3 TEA, corresponding to ~1.8 nM. In similar experiments with different E:T cell ratios, we observed that both HL60 CD117 high and CD117 medium cells could be quantitatively killed at E:T ratios as low as 1:1, while the killing of CD117 low cells required a higher density of T cells. The biocidal effect on non-transduced HL60 cells was negligibly low, confirming the requirement of a simultaneous engagement of CD117 and CD3 for specific killing. We repeated the same experiment with an engineered MOLM14 cell line, which also expressed CD117 at comparable high levels, incubating the target cell line with human T cells at an E:T of 1:1 for 24, 48 or 72, 120 or 192 hours. Complete killing of the target cell line was achieved at 120 and 192 hours and after supplemental addition of T cells and CD117xCD3 TEA at 72 hours (see example figure). Experiments with primary cells (HSPCs from healthy donors or blast cells from AML patients) at an E:T of 1:1 confirmed specific killing of target cells in an antigen-density- and concentration-dependent manner after 48h.
CONCLUSIONS: We have generated a novel bispecific antibody, which binds to human CD117 (expressed on HSCPs and AML/MDS blast cells) and to CD3 (expressed on T cells), which we term CD117xCD3 TEA. The antibody induces selective T cell-mediated killing of cell lines with different surface levels of CD117, as well as of healthy HSPCs and primary human AML cells. Thus, the newly generated CD117xCD3 TEA might be developed clinically in order to erradicate residual AML/MDS and at the same time serve as a milder preconditioning approach prior to allo-HSCT in frail AML/MDS patients.
Kiefer: ETH Zurich: Current Employment, Patents & Royalties: CD117xCD3 TEA. Myburgh: University of Zurich: Patents & Royalties: CD117xCD3 TEA. Guggisberg: F. Hoffmann-La Roche AG: Current Employment. Abdelmotaleb: F. Hoffmann-La Roche AG: Current Employment. Mock: Philogen S.p.A.: Current Employment. Neri: Philogen S.p.A.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Multiple patents on vascular targeting; ETH Zurich: Patents & Royalties: CD117xCD3 TEA. Manz: University of Zurich: Patents & Royalties: CD117xCD3 TEA; CDR-Life Inc: Consultancy, Current holder of stock options in a privately-held company.